By decision of the Director General of the French Agency for the Safety of Health Products dated April 12, 2012:
Considering highlighted by the survey conducted by the French Agency for the Safety of Health Products (AFSSAPS) of November 2006 to end of April 2007, on a sample of more than 200 pharmacies identified as having significant activity preparation, practice prescription compounding for weight loss;
Whereas preparations referred slimming identified in This investigation consisted in particular of the following plants: Citrus aurantium L. ssp aurantium (Citrus aurantium L. ssp amara), Garcinia cambodgia or Hoodia gordonii;
Whereas the requirements of preparations through a process of weight loss have shown that they carry risks;
Considering the adverse cardio- serious vascular observed following consumption of products of Citrus aurantium L. ssp aurantium (Citrus aurantium L. ssp amara or green fruit of the bitter orange or citrus aurantium) in Canada, namely tachycardia, transient collapse, ventricular fibrillation and unconsciousness;
Whereas the green fruit of Citrus aurantium L. ssp aurantium (Citrus aurantium L. ssp amara) contains synephrine, alpha-adrenergic agonist friendly and pharmacologically related to ephedrine for use in humans is banned in France since October 2003 due to his serious adverse cardiovascular effects vascular and neurological, that, due to its pharmacological properties, synephrine may cause adverse effects serious cardiovascular similar to those associated with the use of ephedrine;
Garcinia cambogia, also known guttier, is a small Asian shrub has long been known for the gum that is extracted from the resin (gamboge).
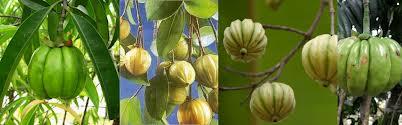
Recent research has shown that the skin of the fruit contained hydroxycitric acid (HCA), a fruit acid that proves to be very effective in regulating appetite and body weight.
It acts within the cell, by inhibiting the production of enzymes usually responsible for the conversion of sugars into fat.
Garcinia allows a reduction in the size of fat cells, decreased food consumption and sweet cravings and even lower cholesterol.
Our Garcinia cambogia is titrated to 60% in hydroxycitric acid (HCA). This guarantees exceptional titration an important activity, so the best efficiency.
Whereas, because of its properties pharmacological related to the presence of synephrine, green fruit of Citrus aurantium L. ssp aurantium (Citrus aurantium L. ssp amara) meets the definition of the drug according to
Whereas the use of preparations of green fruit of Citrus aurantium L. ssp aurantium (Citrus aurantium L. ssp amara), based Garcinia cambodgia or Hoodia gordonii, through a process of weight reduction has proven risks in relation to a benefit that is not established on a map therapeutic;
Considering that it follows from the above that the administration to humans in the form of compounding, officinal and hospital, including homeopathic preparations, referred slimming, composed of green fruit of Citrus aurantium L. ssp aurantium (Citrus aurantium L. ssp amara), composed of Garcinia cambodgia or Hoodia gordonii and the administration to humans of Garcinia cambodgia and green fruit of Citrus aurantium L. ssp aurantium (Citrus aurantium L. ssp amara) is likely to constitute a serious threat to human health in relation to therapeutic justifications can not be held established, it is necessary, therefore, to prohibit the importation , preparation, prescription and dispensing of these preparations as well as the prescription, dispensing and administration to man the plant Garcinia cambodgia and green fruit of Citrus aurantium L. ssp aurantium (Citrus aurantium L. ssp amara)

